Type 1 Diabetes (T1D)
Overview
- Type 1 Diabetes is an autoimmune disorder caused by destruction of insulin-producing beta-cells in the pancreas from the patient’s own immune system.
- Anyone can get it, but it is most often diagnosed in young children with an average age at diagnosis of 13 years old with increased risk if a parent has been diagnosed with the disease.
- It is a lifelong disease with no current cures and many life-altering implications.
Type 1 diabetes (T1D) is a chronic, lifelong severe autoimmune condition where the pancreas stops producing insulin, the hormone that allows sugar (glucose), our body’s main source of energy, to enter our cells. Without insulin, the body can’t use glucose for energy and too much sugar builds up in the bloodstream which leads to hyperglycemia.
Left untreated for long periods of time, high blood sugar is damaging to the body and can affect the vessels that supply blood to vital organs, increasing the risk of heart disease, stroke, vision problems, nerve problems and kidney disease. T1D currently requires daily administration of insulin to replace the insulin the body is not making itself.
Common symptoms of T1D include increased thirst, frequent urination, hunger, fatigue, unexplained weight loss and blurred vision.
While the precise cause of T1D is unknown, experts usually point to an abnormal autoimmune reaction that causes the body’s own immune system to destroy beta cells, the cells in the pancreas that are
responsible for making insulin and releasing it into the body. This reaction can go on for months or even years before any T1D symptoms appear.
Type 1 diabetes is usually diagnosed in children, teens, and young adults, but can onset in people up to 45 years old. Certain genes may increase the risk of T1D, especially when combined with certain environmental triggers, such as a viral infection.
Unlike type 2 diabetes, T1D is not caused by diet and lifestyle habits, but diagnosis and disease management can profoundly affect lifestyle and quality of life for children and adolescents – and their parents or caregivers – due to the need for careful blood glucose monitoring, dietary restrictions and risk of serious complications.
While there is no way of preventing T1D yet, disease modifying drugs (targeted biologics) may help postpone onset or delay progression by slowing down the immune system’s ability to attack insulin-producing pancreatic beta cells.
Prevalence
- Since the year 2000, T1D prevalence has increased at four times the rate of global population growth1
- An estimated 8.4 million people were living with Type 1 Diabetes around the globe in 20212
- This number is predicted to increase to 13.5-17.4 million people living with T1D by 20402
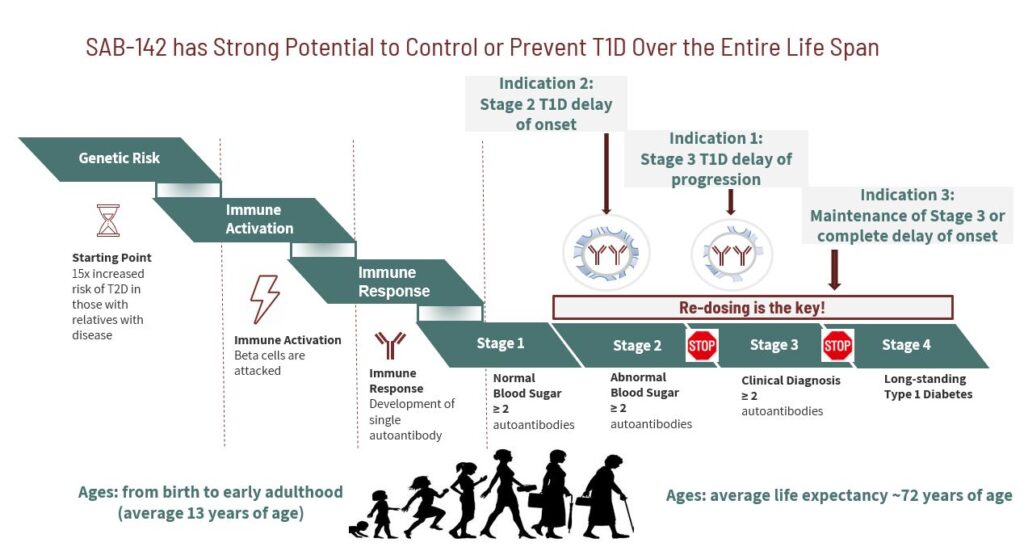
Stages of Type 1 Diabetes
According to the NIH, the stages of T1D are as follows: 11
- Stage 1: Presence of beta-cell autoimmunity – pre-symptomatic
- Stage 2: Presence of beta-cell autoimmunity along with an abnormality in blood sugar stability (dysglycemia) – pre-symptomatic
- Stage 3: Full clinical onset of disease symptoms
Shifting T1D Treatment from Chronic Disease Management to Disease-Modifying Therapies
Type 1 Diabetes is complex and requires daily, sometimes hourly intensive management and treatment of the disease and its numerous complications. Most T1D treatment options today revolve around insulin management, with the goal of maintaining normal blood sugar levels, such as by eating healthy foods and frequent blood sugar monitoring.
Despite advancements in glucose monitoring and insulin administration, mortality among T1D patients remains 5 to 13 times higher compared to matched controls without T1D.
To prevent these millions of lives from being lost, treatment strategies must shift from chronic disease management to true disease-modifying therapies.
Targeted gene therapies are being explored to address potential genetic causes underlying the autoimmune destruction of beta cells to restore insulin production and potentially delay or halt the onset of T1D. There have also been trials showing a delay in progression of recent onset of T1D using rabbit anti-thymocyte globulin (rATG).
SAB-142: Disease Modifying Treatment
New disease-modifying therapies come slowly for T1D, but two decades of work has advanced the clinical proof of concept for rATG in treating T1D and this work serves as a springboard for a streamlined and efficient development program leveraging SABs Diversitab platform to rapidly produce SAB’s T1D investigational treatment, SAB-142, a first-in-class fully-human polyclonal antibody treatment with a clinically validated mechanism of action (MoA) and superior efficacy in delaying onset of clinical Stage 3 Type 1 Diabetes (T1D).
