SAB Biotherapeutics Program Targeting COVID-19: SAB-185
Coronavirus disease 2019 (COVID-19) is caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) virus, a highly mutating virus in humans causing respiratory illness that can be spread from person-to-person. For most people, COVID-19 resolves on its own and they will not need medical care, recovering in less than two weeks. However, COVID-19 can cause severe illness and complications, particularly among those at high risk including those with underlying medical conditions and adults over the age of 65.
To learn more about COVID-19 click here.
SAB-185’s Targeted Product Profile and Administration Routes:
SAB-185 is a first-in-class fully-human broadly neutralizing polyclonal antibody treatment designed to increase the body’s ability to fight multiple variants of COVID-19, especially in high-risk patients.
SAB-185 is being studied as a therapeutic for high-risk COVID-19 patients defined by the CDC as: 1
- People who are unvaccinated
- Adults 65 years of age and older
- Patients in nursing homes/assisted living
- Pregnant and recently pregnant
- Physically inactive
- Those who smoke (currently and formerly)
- Users of corticosteroids or other immunosuppressive medications
- Those of all ages with underlying medical conditions, including but not limited to:
- Heart disease
- Diabetes
- Lung disease
SAB-185 is administered intravenously, with subcutaneous and intramuscular administration in development.
The Polyclonal Approach to a Frequently Mutating Virus
SAB-185 represents a highly differentiated treatment option that provides an appropriate match against the complexity, diversity, and the mutations SARS-CoV-2 presents.
This novel fully-human multi-epitope binding IgG candidate for COVID-19 treatment may have value both therapeutically, as a treatment for already symptomatic COVID19 patients, and for post-exposure prophylaxis when a person gets exposed to SARS-CoV2 virus but has not yet developed symptoms. SAB-185 is produced without the need for human convalescent plasma, blood donations, or serum. SAB-185 production efficiently produces large volumes of fully human polyclonal antibodies targeted specifically to address SARS-CoV-2.
Unlike monoclonal antibodies, SAB-185 has the capacity to sustain efficacy despite SARS-CoV2 viral mutations protecting against potential escape mutants resulting in new variants (or strains). SAB-185 exhibits a half-life that allows long-term protection against a mutating virus with only a single dose.
As COVID-19 is a frequently mutating virus with multiple documented variants, SAB-185’s cross-reactivity and in vivo efficacy in animals and humans to different strains provides the most logical approach to treat COVID-19. It is the only potential therapeutic amongst biologics that showed sustained efficacy across multiple variants evolved over the last 3+ years.
SAB-185 is a target-specific human immunoglobulin G (hIgG), which are polyclonal antibodies, designed to specifically bind to the SARS-CoV2 viruses. SAB-185 supplies broadly neutralizing antibodies working together with antibodies that are naturally produced by the body’s immune system. SAB-185 antibodies can neutralize many different genetic variants of COVID-19.
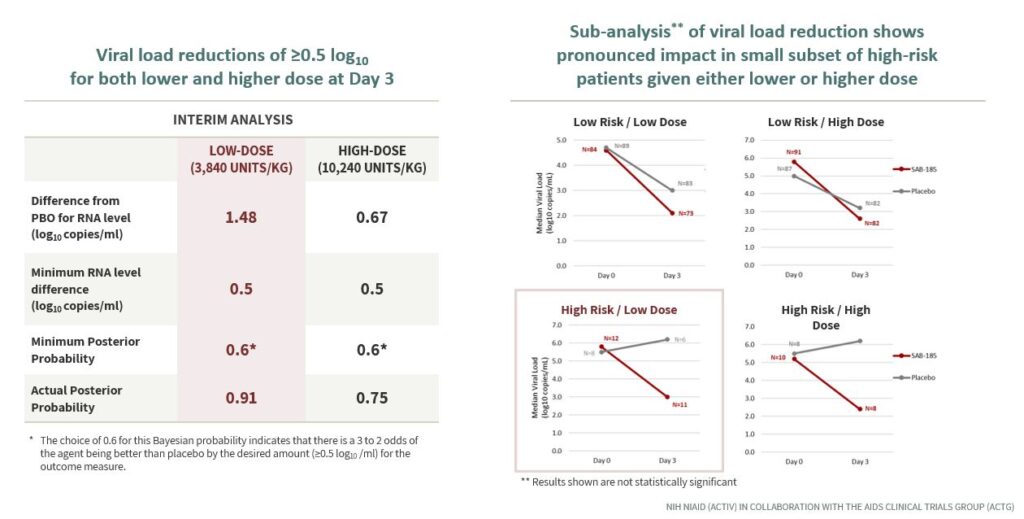
In Phase 2 data from the NIH COVID-19 ACTIV-2 Trial, a study designed to evaluate treatments for COVID-19 in patients at higher risk for progression to hospitalization, SAB-185 showed statistically significant reduction in viral load at day 3 [See Figures 2 & 3]. SAB-185 has also demonstrated its ability to reduce the viral load in patients given higher and lower doses [See Figure 4].
For information on the Phase 3 trial data, click here.
Established Proof-of-Concept for SAB-185:
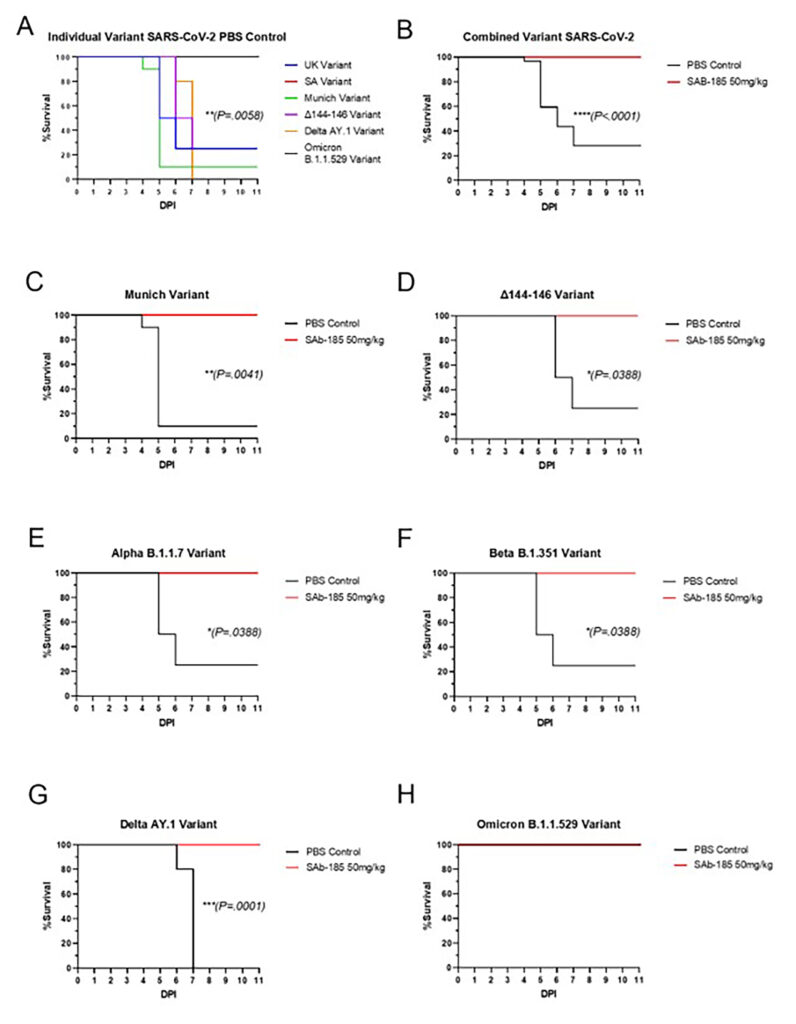
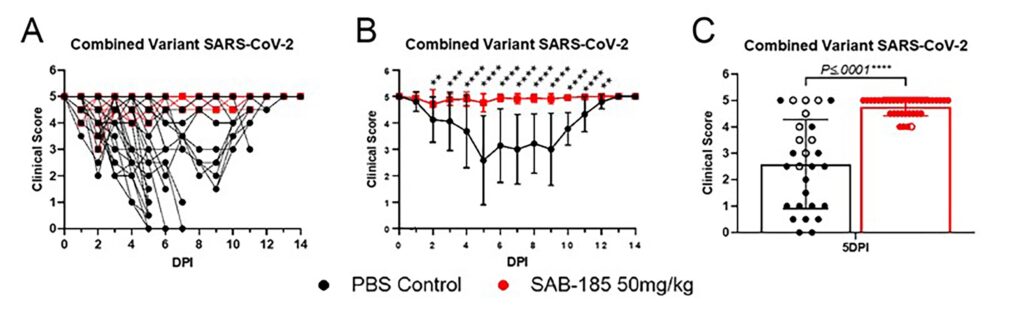
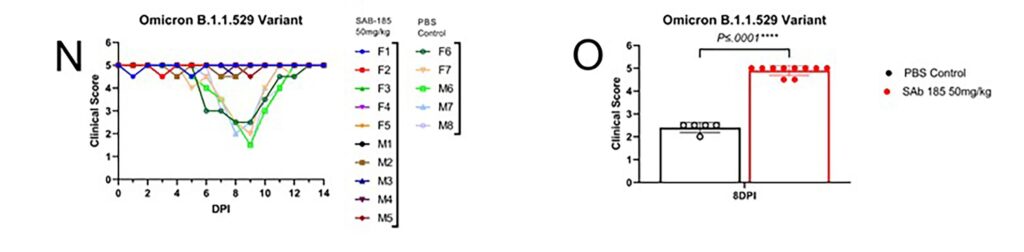
SAB-185 Protects Recombinant hACE2 Hamsters from Mortality and/or Severe Morbidity from SARS CoV-2 Variants Including Omicron
CONCLUSION:
SAB-185 Met Virology Endpoints in an NIH ACTIV-2 Phase 2 Trial: Data Confirms Graduation to Phase 3
SAB Presentation: ADVANCING POWERFUL NEW CLASS OF IMMUNOTHERAPEUTIC ANTIBODIES (sab.bio)
2 https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html
3 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8887070/
4 https://www.cdc.gov/media/releases/2022/s0715-COVID-VE.html