SAB Biotherapeutics Program Targeting Influenza: SAB-176
Influenza (flu) is a common, contagious respiratory viral infection. Influenza can infect the nose, throat, and sometimes the lungs, causing fever, chills, muscle aches, cough, congestion, runny nose, headaches and fatigue. Most people who get influenza will quickly recover on their own, but it can sometimes cause serious illness and even death – particularly for those in high-risk groups, including but not limited to those with chronic conditions or weakened immune systems and adults over age 65. To learn more about Influenza click here.
SAB-176’s Targeted Product Profile and Administration Routes:
SAB-176, an investigational drug, is a fully-human polyclonal antibody being studied for treatment and post-exposure prophylaxis of pandemic and seasonal influenza in patients who are at high-risk for severe influenza outcomes.
SAB-176 is being studied as a therapeutic for patients at risk for severe influenza outcomes defined by the CDC as:
- Adults 65 years of age and older
- Immunocompromised due to a disease or medications (autoimmune, cancer, etc.)
- Patients with respiratory, cardiovascular, kidney, metabolic, neurological disorders
SAB-176 administration routes:
- Intravenous
- Subcutaneous and Intramuscular administration in development
Why We Need New Treatments for Influenza
While vaccination is currently the best way to prevent and/or minimize the effects of influenza, the vaccine development process and the process of choosing the proper circulating strains, combined with the mutating nature of the virus, present unique challenges. It’s also important to note that about half of the population doesn’t get the flu vaccine, with 54.7% of the adult population during the 2018-2019 season not getting a flu vaccine. In terms of therapeutics, only a few have been launched to address the flu and have resulted in less-than-ideal clinical success.
The current influenza vaccine manufacturing process can take five to seven months, from the time the World Health Organization (which chooses the influenza viruses to be included in the annual vaccine) to the time the vaccine is developed and administered to patients. For a virus that is constantly undergoing genetic mutation, this long lag time in vaccine development can lead to “drifts,” or small alterations of the influenza virus’s surface proteins, making it more difficult to match influenza vaccines to the prevailing seasonal strain circulating months later. Abrupt mutations result in antigenic “shifts”, creating novel influenza viruses capable of causing global or pandemic influenza vs. seasonal strains of influenza.
A combination of influenza virus mutations resulting in antigenic drift or antigenic shift and a time lag between virus selection and vaccine availability, make it more difficult for those vaccines to elicit an antibody response to match the virus that will be circulating in the upcoming season. This method means that the virus could be just one or two mutations away from rendering that year’s vaccine ineffective.
To be clear, as of today, getting an annual influenza vaccination is still the most efficient way to protect oneself against infection and illness caused by the virus.
Influenza’s continual mutation is why there is a need for better, more effective influenza vaccines and for more effective therapeutic treatments. CDC data show that over the last 13 years, the annual influenza vaccine’s effectiveness ranged from as low as 19% and up to 60% at its highest (between 2009 and 2021). The low level of efficacy of the annual vaccine often means that most people are at risk of contracting the disease. Those at highest risk of developing severe complications from influenza, such as the elderly, those who are immunocompromised (a weakened immune system), children under the age of 2, and pregnant women are most critically affected. New studies have shown that influenza vaccine effectiveness is significantly lower among immunocompromised adults compared with non-immunocompromised adults.
Similarly, antivirals, the current treatment option available for influenza, only work best when taken within 48 hours of onset of flu symptoms. Even approved antiviral small molecule treatments, while they may shorten duration of fever and symptoms, are marginally effective against clinically meaningful endpoints or may result in virus mutations resulting in the antiviral-treatment resistant influenza strains. Antiviral drug resistance is an increasing concern in immunocompromised patient populations, where ongoing viral replication and prolonged drug exposure lead to the selection of resistant strains.
An Innovative, Polyclonal Approach to a Frequently Mutating Virus
Figure 1: SAB-176 Key Differentiators
SAB’s investigative polyclonal therapeutic, SAB-176, is an anti-type A and type B immunoglobulin for the treatment of influenza disease and the first fully-human broadly neutralizing polyclonal antibody therapeutic intended to prevent or reduce severe outcomes of influenza infection in patients at high risk for severe complication, including those who are immunocompromised. SAB-176 supplies broadly neutralizing antibodies, in addition to antibodies generated by one’s immune system.
These fully human antibodies can neutralize many different variants of influenza A and B. SAB-176’s recently completed clinical proof-of-concept Phase 2A trial showed statistically significant reduction in viral load, confirming high cross-reactivity (the ability to recognize and react to strains it was not specifically produced against) to the pandemic influenza strain (not targeted with immunogen to produce SAB-176 treatment) in humans. SAB’s fully human polyclonal antibodies achieved both statistically significant reduction in viral load and statistically significant improvement in symptomology at Day 4, confirming SAB-176’s favorable safety and tolerability profile. [See Figure 3 below]
SAB-176 has consistently demonstrated the ability to be adaptive and cross-reactive to mutating strains of influenza in pre-clinical in vivo and in vitro studies. SAB-176 has demonstrated its ability to protect against various influenza strains through its higher titers (concentration) relative to those achieved by annual vaccination. [See Figure 2 below]
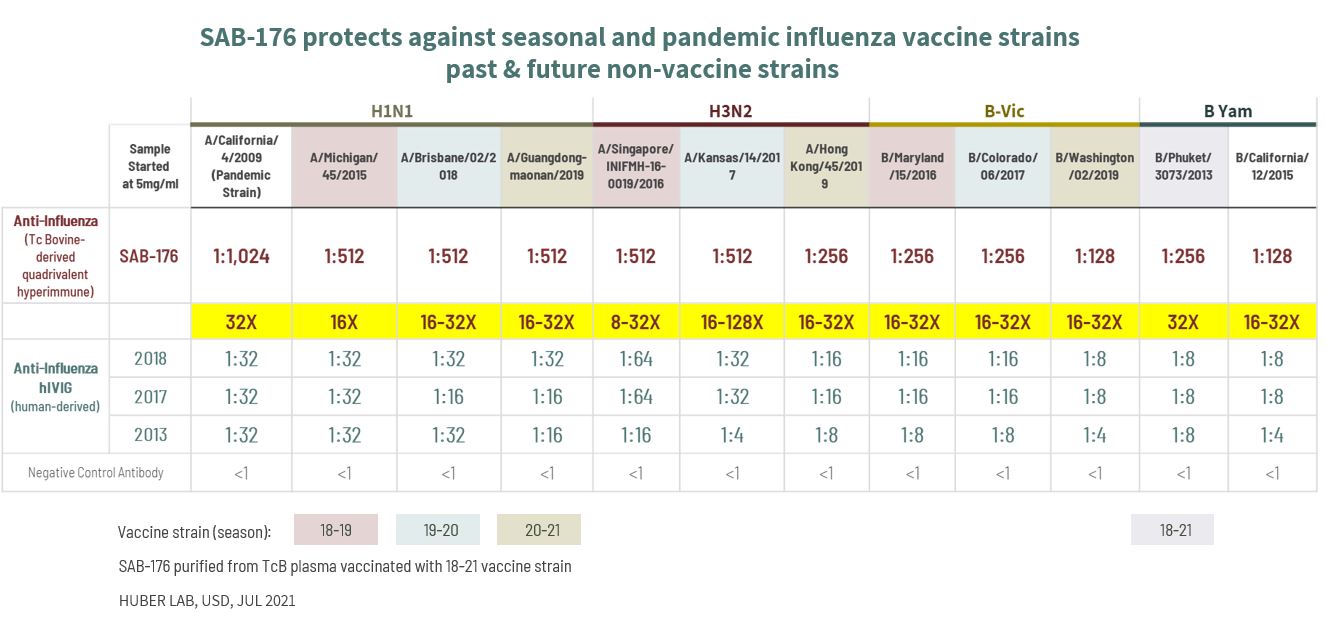
Figure 2: SAB-176’s Protection Against Seasonal and Pandemic Influenza Strains
Unlike monoclonal antibodies, polyclonal antibodies have the capacity to sustain their efficacy despite viral mutations. Due to their homogeneity, high specificity to a single epitope and their low degree of cross-reactivity, monoclonal antibodies are not positioned to treat influenza as well as polyclonal antibodies are. SAB’s polyclonal antibodies exhibit broad neutralization across multiple epitopes of Influenza A and B viruses. SAB-176 has a half-life that allows long-term protection against a virus and its mutations with a single dose. They are fully-human antibodies, which means they have a low risk of triggering the production of anti-drug antibodies throughout the treatment cycle, a phenomenon well known in non-human antibody treatments and associated with a loss of efficacy and side effects for patients.
Established Proof-of-Concept for SAB-176:
CONCLUSION:
Tc Bovine-derived Fully Human Polyclonal Antibody Treatment for Superior Long-lasting Efficacy for Prophylaxis and Management of Influenza Met Primary Endpoint of Viral Load Reduction in Phase 2a Challenge Study
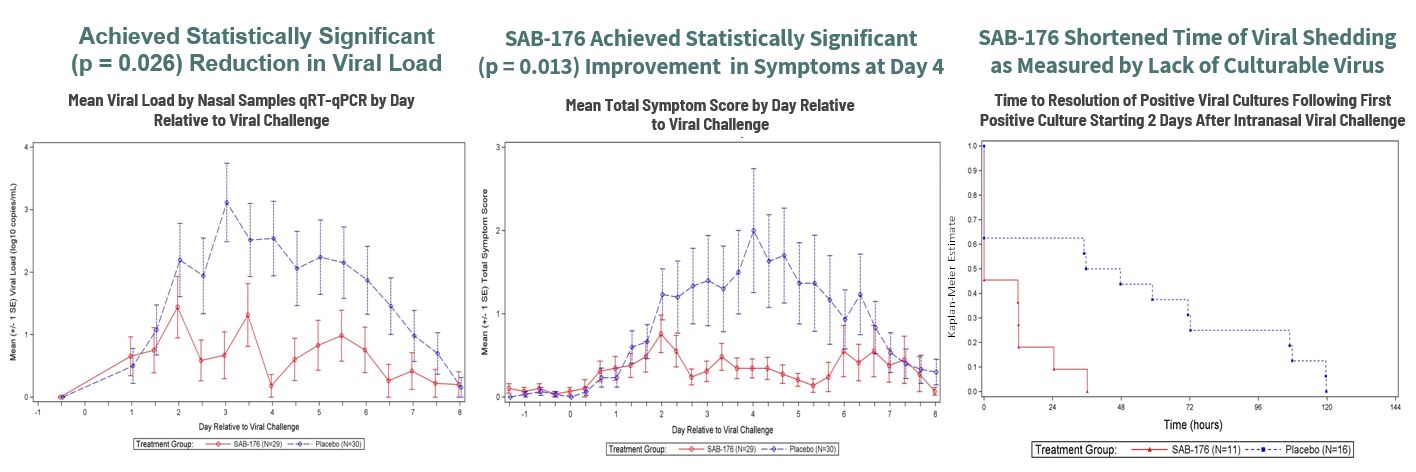
Figure 3: A Phase 2a, Randomized, Double-Blind Trial in H1N1 Challenged Adults
Reference:
SAB Presentation: ADVANCING POWERFUL NEW CLASS OF IMMUNOTHERAPEUTIC ANTIBODIES (sab.bio)