SAB BIO is a clinical-stage biopharmaceutical company focused on developing multi-specific, high-potency, human immunoglobulin G (hIgG) to treat and prevent immune and autoimmune disorders. The Company was founded in 2014 and traded on Nasdaq (SABS) since 2021.
Using advanced genetic engineering and antibody science, SAB BIO developed a proprietary technology which holds the potential to generate additional novel therapeutic candidates utilizing the human immune response, without the need for human donors or convalescent plasma. SAB BIO has optimized genetic engineering in the development of transchromosomic cattle, or Tc-Bovine™, to produce hIgG. SAB BIO’s drug development production system is able to generate a diverse repertoire of specifically targeted, high-potency, hIgGs that can address a wide range of serious unmet needs in human diseases.
To date, SAB BIO has conducted seven clinical trials that have dosed 700 individuals across multiple therapeutic areas. The safety and immunogenicity database for these trials shows zero patients with serum sickness or neutralizing anti-drug antibodies, which suggests an improved safety profile over other therapies.
SAB BIO’s lead product candidate, SAB-142, targets autoimmune Type 1 Diabetes (T1D) with a disease-modifying therapeutic approach that aims to change the T1D treatment paradigm by delaying onset and potentially preventing disease progression of Stage 3 T1D patients.
SAB-142: Potential Disease-modifying Immunotherapy Being Developed to Delay the Onset and Progression of Type 1 Diabetes
The company’s lead investigational candidate, SAB-142, is a multi-specific, fully human anti-thymocyte globulin (hATG) disease-modifying immunotherapy to delay the onset and progression of Stage 3 type 1 diabetes (T1D).
In the Phase 1 clinical trial of SAB-142, called the HUMAN Trial (*HUman anti-thymocyte biologic in first-in-MAN clinical study), the data confirmed a superior safety profile enabling outpatient dosing and redosing potential.
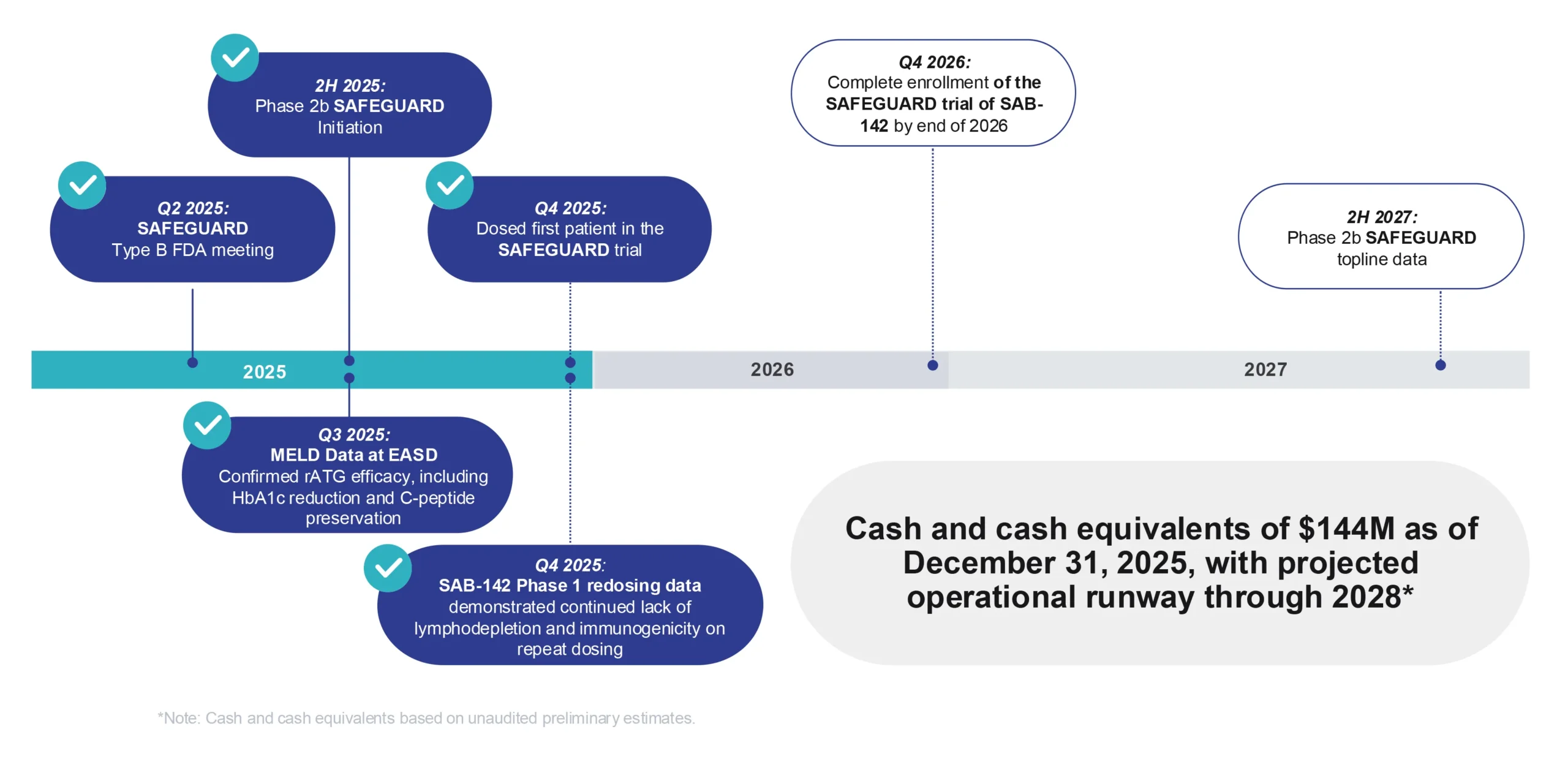
The Phase 1 data also confirmed advancement into a registrational Phase 2b study in newly diagnosed adult, adolescents, and pediatric T1D patients (age 5-40), called the SAFEGUARD trial (SAFety and Efficacy of human anti-thymocyte immunoGlobUlin SAB-142 ARresting progression of Type 1 Diabetes).
SAB BIO initiated the multicenter, globalSAFEGUARD trial in 2025, and patient dosing is underway.
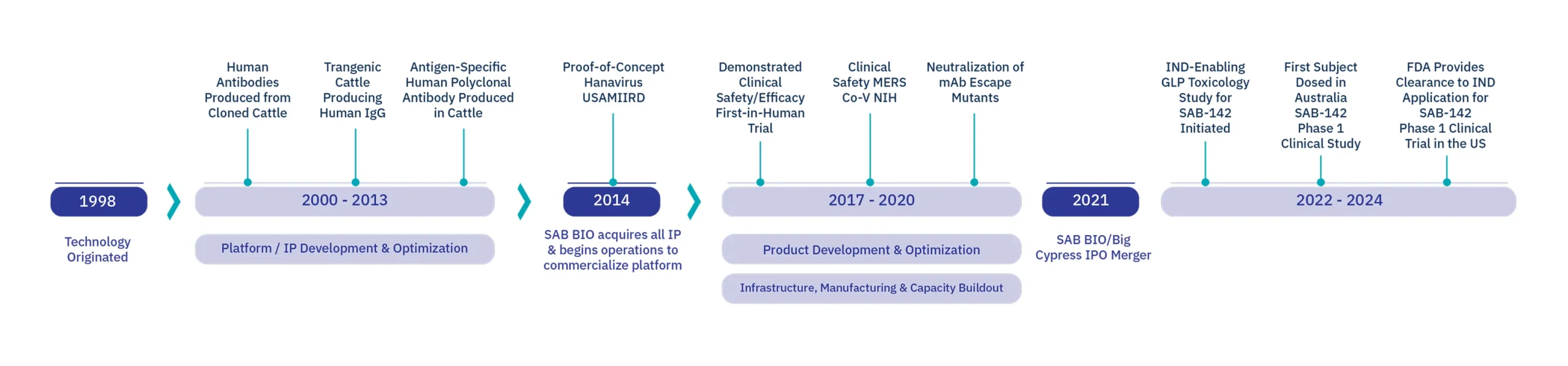
Company History and Platform Evolution
Strong 2025 Execution with Significant Catalysts in 2026-2027